Finally, the Sinovac COVID-19 study is released and the World Health Organisation has approved it for use.
Read more about Sinovac clinical trials: https://www.who.int/news-room/events/detail/2021/04/29/default-calendar/extraordinary-meeting-of-the-strategic-advisory-group-of-experts-on-immunization-(sage)-29-april-2021
How was the Sinovac Phase I and Phase II conducted?
I will simplify the Sinovac Phase I and Phase II study in the shortest way possible because I think that we should put more emphasis on Phase III trials.

Phase I and Phase II trials (or called the COVIV-01) is a double-blind placebo-controlled study. This study is conducted in subjects from age to 18 years old to 80 years old. The subjects were given a few variants of dosages:
2 doses on Day 0, 14
2 doses on Day 0, 21
2 doses on Day 0, 28
3 doses on Day 0, 21, 42.
3 doses on Day 0, 28, 56.
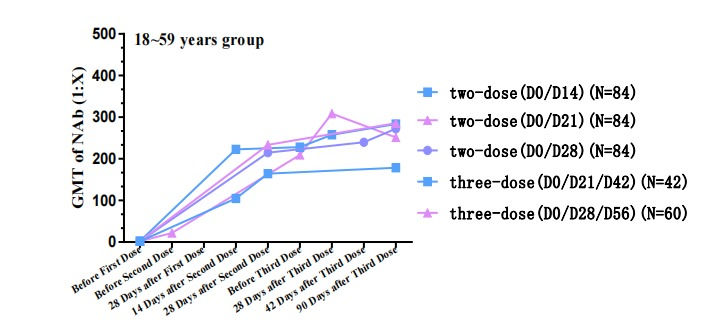
The level of neutralizing antibody after two or three doses of immunization is significantly superior to that after one dose of immunization. In the two-dose immunization schedule, the neutralizing antibody level at Day 0,21 and Day 0, 28 schedules are significantly better than that at Day 0, 14. Subjects' antibodies were monitored until 90 days after the last dose and it has been concluded that there is no significant neutralizing antibody reduction observed until Day 90 after the last dose in all age groups.
Is the Sinovac vaccine safe during the Phase I and Phase II trials?
Predominantly the vaccine has not caused and lethality or death. There is certainly a common reaction ranging from local, systemic and severe adverse reaction to which anyone can be affected with any other vaccines.

Overall, the vaccine adverse reactions studied in Phase I and Phase II trials have been shown to be minimal, and severe adverse reactions are unrelated to the vaccine.

How many centers are conducting Phase III trials is for Sinovac?
The Phase III trial is a multicenter study in a few countries and the trial as are follows:
COVIV-02 (Sponsored by the China National Biotec Group Company in Bahrain, Egypt, Jordan & UAE)
COVIV-03 (Sponsored by Universidad Peruana Cayetano Heredia, Peru)
COVIV-04 (Laboratorio Elea Phoenix S.A, Argentina)
COVIV-05 (Beijing Institute of Biological Products Co., Ltd, China)
To date, only the COVIV-02, COVIV-03, and COVIV-05 have interim results available.

*BIBP (VACCINE GROUP 1) is another vaccine developed by China is the main focus of the study.
*WIBP (VACCINE GROUP 2) is another vaccine developed by China and not evaluated here. This WIBP is an investigational vaccine based on another type of SARS-CoV-2strain.
What are the effective rates of the Sinovac vaccine?
The CoronaVac (Sinovac) vaccine was 100% effective in preventing COVID-19 sufferers from being hospitalized or dying. 83.7% effective in avoiding cases that required any medical treatment, but only 50.65-75% effective at keeping people from getting infected.
Some people will prefer to get the Pfizer or the AstraZeneca Vaccine due to the higher effective rates as compared to the CoronaVac. There may be some elements that you will need to consider before making your judgment over the efficacy of the different vaccines.
I want to put it into context for you to understand the main point! Some of these vaccine-producing companies did their study in developed countries with good sanitary conditions, medical services, social welfare, and lower population density per square (density of people and a living area). These conditions reduce the risk of infection in the population therefore it produces a higher effective rate as compared to CoronaVac.
You will notice that CoronaVac's effective rates are inconsistent from country to country:
Brazil 50.4%
Chile 67%
Uruguay 61%
Turkey 91.25%
Indonesia 94%
CoronaVac did their studies in the third world, developing, and developed countries. Some of the countries had poor sanitary conditions, medical services, social welfare, and higher population density per square (density of people and a living area). These conditions increase the risk of infection in the population therefore the CoronaVac's effective rate to reduce infection is lower than other COVID vaccines. This does not mean that the CoronaVac is not effective. It appears less effective due to the conditions it was subjected to.
At the end of the day, all these vaccines have one thing in common which is 100% rates of hospitalization. Hospitalization means that the disease is severe and all brands of the vaccines have prevented 100% hospitalization.
Is the Sinovac vaccine effective in the Phase III trial?
Interim data means data that is collected during the period of intervention. So the interim data on 31/12/2020 shows the recruitment of 41301 subjects in the study.
The study was registered at clinicaltrials.gov (NCT04510207).
In COVIV-02, serum samples were collected from all participants 14 days after the second dose. A subset of participants was enrolled into an immunogenicity subgroup (~900 per site) to assess the antibody response on days 14, 28, 90, 180, and 360 after the second dose. Seroconversion 14 days after the second dose was defined as a 4-fold increase in neutralizing antibody titer compared with baseline and 99.3% of COVID-19 vaccine group seroconverted with good antibody titer as compared to 2.3% in the placebo group. The COVID-19 vaccine group in 18-59 years old participants had higher neutralizing antibodies as compares to participants above the age of 60 years old.
Interpretation:
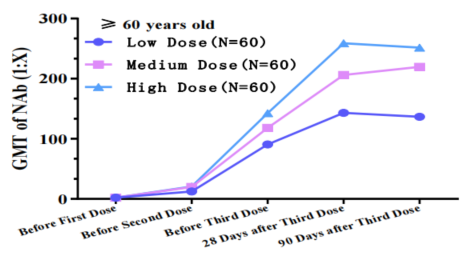
The COVIV-02 study shows that completion of 2 injections of the COVID-19 vaccine is able to produce good rates of seroconversion and neutralizing antibody against COVID019 virus. All age groups from 19 years old and above had good seroconversion but subjects from 19-59 years old have better levels of neutralizing antibody if compared against those above the age of 60 years olds. Regardless of the level of neutralizing antibody levels; all age groups in the study had adequate antibodies against the COVID-19 virus after they have completed their vaccinations.
ADDITIONAL CLINICAL INFORMATION (for Doctors):
In the table above. 41301 subjects (as of 31/12/2021) were recruited into the phase III trial and 40408 subjects were 18-59 years old and 893 subjects were >60 years old. Of the total 41301 subjects, 85% of the participants were male and 15% were female.
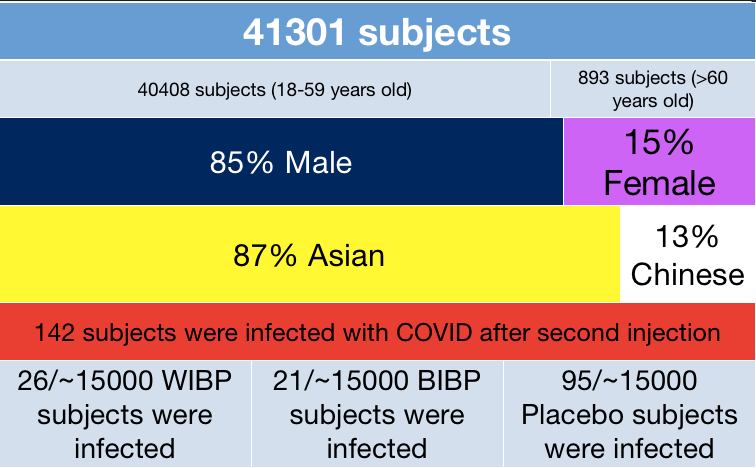
*41301 subject recruited as of 31/12/2021
*BIBP (~15000 subjects) is another vaccine developed by China is the main focus of the study. *WIBP (~15000 subjects) is another vaccine developed by China and not evaluated here. This WIBP is an investigational vaccine based on another type of SARS-CoV-2strain.
After a follow-up of about 150 days from the time of vaccination; 142 subjects became infected with the COVID-19 virus (26 subjects in the WIBP group, 21 subjects in the BIBP group, 95 subjects in the placebo group respectively).
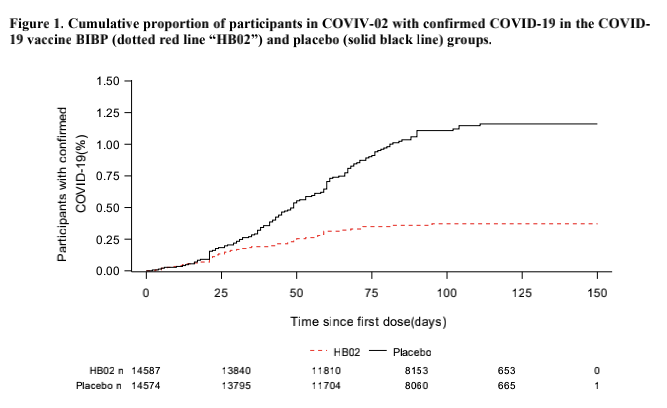
In the BIBP group (HB02 in red dotted line) -----, the actual number of participants was 14587 subjects versus 14574 subjects in the Placebo group (straight line). The graph shows a higher incidence of infective rate in the placebo group as compared to the BIBP group.
Interpretation:
The BIBP vaccinated group shows better good efficacy in preventing infection among the population studied.
Is the Sinovac vaccine suitable for people living with HIV?
HIV subjects were excluded in the COVIV-2 study therefore we do not have sufficient data about people living with HIV. As a rule, we tend to observe the general rules of vaccination in people living with HIV:
Make sure that you are stable HIV:
Viral load <50 copies/mL (within 6 months)
CD4 count >200cell/mm3 (within 6 months)
On treatment (> 6 months)
Sinovac (China vaccine) is a killed vaccine and should be relatively safe. I personally will advise that you should achieve stable HIV conditions before taking the vaccine. I do not intend to misguide or oppose the use of COVID-19 vaccines, but I would like to achieve HIV stable conditions before giving the vaccine.

If your immunity or CD4 count is too low; you may not evoke a good response to the vaccine while subjecting yourself to the possible adverse effects of the vaccine. Since the vaccine is still new, there are many variables and side effect which we do not know about. Therefore, I believe your doctor and you should sit down to discuss your vaccination options and your treatment plans especially if that person has not achieved stable HIV conditions.
Get the fact right for you to make the best decision for yourself.

How many doses of the CoronaVac is required to achieve full immunisation?
The CoronaVac (Sinopharm, China) requires 2 doses which are given on Day 0 and the second dose can be given from Day 14 to day 28.
Is the Sinovac vaccine safe in the Phase III trials?
Yes. The CoronaVac Vaccine in the phase III trials have been published. It shows that is safe however there are some reactions are as follows:
Local reactions
Erythema (redness)
Pruritus (Itching)
Pain
Swelling
Induration (hard nodule at the injection site)
Systemic reactions
Nausea
Fever
Vomiting
Fatigue
Maculopapular rash
Constipation
Erythematous rash (red rash)
Pruritus (itching)
Loss of appetite
Headache
Diarrhea
Arthralgia (joint ache)
Myalgia (muscle ache)
Severe adverse reactions - These severe adverse reactions have been scrutinized and researchers are unable to see the correlation of the Sinovac vaccine to the diseases stated below.
Infection
Metabolic and nutritional diseases
Musculoskeletal and connective tissue disease
Congenital diseases
Various injuries, poisoning, surgical complications
Endocrine system diseases
Kidney and urinary system diseases
Surgical and medical operations
Respiratory, thoracic and medical operations
Reproductive systems and breast disease
Benign, malignant and unknown tumours
Gastrointestinal diseases
Nervous system disease
Heart disease
Vascular (thrombotic event) and lymphatic diseases
What are the take-home message and the summary of the COVID-19 vaccine and people living with HIV?
Make sure that you are stable HIV:
Viral load <50 copies/mL (within 6 months)
CD4 count >200cell/mm3 (within 6 months)
On treatment (> 6 months)
Sinovac (China vaccine) is a killed vaccine and should be relatively safe.
Most COVID-19 vaccines are not live-vaccine and should be safe for people living with HIV.
Do not switch or change your vaccine type until you have completed your dose. If you are using the Sinovac vaccine then stick to the Sinovac vaccine
Check your MySahjetera App for updates about your turn for vaccination (Available in Malaysia only) or you can register yourself and your loved ones here.
If you have taken the vaccine and suffer from an adverse reaction to the vaccine. Use the MySahjetera App to report the adverse reaction or ask your family doctor to make a manual adverse reaction report to the Jabatan Pharmacy Malaysia (Department of Pharmaceutical Malaysia) Link: https://npra.gov.my/index.php/en/consumers/reporting/reporting-side-effects-to-medicines-conserf-or-vaccines-aefi-2.html
Frequently asked questions about the Sinovac Vaccine:
Is the Sinovac Vaccine safe in children below 18 years old? Currently, there are ongoing studies in paediatrics but only in Phase II. Details will be published later.
Is the Sinovac vaccine safe in pregnancy? We only have animal studies to support the safety of the vaccine in pregnancy but there is no evidence supporting the Sinovac vaccine safety in human studies.
Should I test myself for pregnancy before taking the Sinovac vaccine? Currently, there are no recommendations to check for pregnancy before taking the vaccine. A urine pregnancy test should not be a routine screening.
If I am pregnant after taking the first Sinovac vaccine dose, should I continue taking the second Sinovac vaccine dose? We currently do not recommend taking the second Sinovac vaccine dose. After the first dose may already be able to provide some immunity against the COVID-19 virus.
Is the Sinovac Vaccine safe in lactating mothers? Data are not available on the potential benefits and risks of the vaccine to breastfed children. However, as the COVID-19 vaccine, BIBP is not a live virus vaccine, it is biologically and clinically unlikely to pose a risk to the breastfeeding child, WHO does not recommend discontinuing breastfeeding because of vaccination.
Should people who had recovered from COVID-19 take the Sinovac vaccine? Available data show that in 6 months after initial natural infection; reinfection is uncommon. People who had recovered from the COVID-19 disease usually have natural antibodies persisting for about 6 months. They can choose to delay the vaccine up to 6 months and vaccinate after about 6 months from the initial onset of the COVID-19 infection when the natural antibodies are reducing. This vaccine can be considered as a "booster vaccine" to induce another antibody peak.
Can people with acute COVID-19 infection take the Sinovac vaccine? WHO does not recommend people to take the vaccine when they are infected with acute onset COVID-19 disease and PCR positive for COVID-19.
Can people who were treated with passive antibody therapy for COVID-19 infection take the Sinovac vaccine? Currently, there are no data on the safety or efficacy of vaccination in persons who received monoclonal antibodies or convalescent plasma as part of COVID-19 treatment. As a precautionary measure, vaccination should be deferred for at least 90 days to avoid interference of the antibody treatment with vaccine-induced immune response.
I have taken the first dose of another brand of the vaccine but I developed an adverse reaction. Can I opt to take the Sinovac vaccine or another vaccine? Currently, there is no data on the safety or efficacy of vaccination in persons who received one type of vaccine and are able to switch in between other types of vaccines. As a precautionary measure, vaccination should be deferred for at least 90-180 days to avoid interference with vaccine-induced immune response.
How is COVID-19 disease graded in Malaysia and China?
Malaysia and China have a slight difference in ways of grading the COVID-19 disease. The first difference is the terminology of the clinical classification; Malaysia uses the term "Category 1-5" and China uses the term " Mild-Moderate-Severe-Critical" The Malaysian grading is easier to be used as compared to the China grading because the China grading is more technical with a few additional criteria. Example: Malaysia Category 4 grading generalises the grade into lung infection with x-ray changes and need of oxygen vs China Severe grading requires doctors or clinicians to complement severity with laboratory results in order to grade the disease. In both Malaysia's and China's severity grading terminology and criteria may sound different but both Category 4 (Malaysia) and Severe (China) grade results in the same end-point which requires oxygen supplementation.

Read more about COVID-19 severity grading in Malaysia: http://covid-19.moh.gov.my/semasa-kkm/122020/treatment-performance-of-covid-19-patients-in-malaysia
Read more about COVID-19 severity grading according to Sinovac clinical study in the image below:


Comments